About us
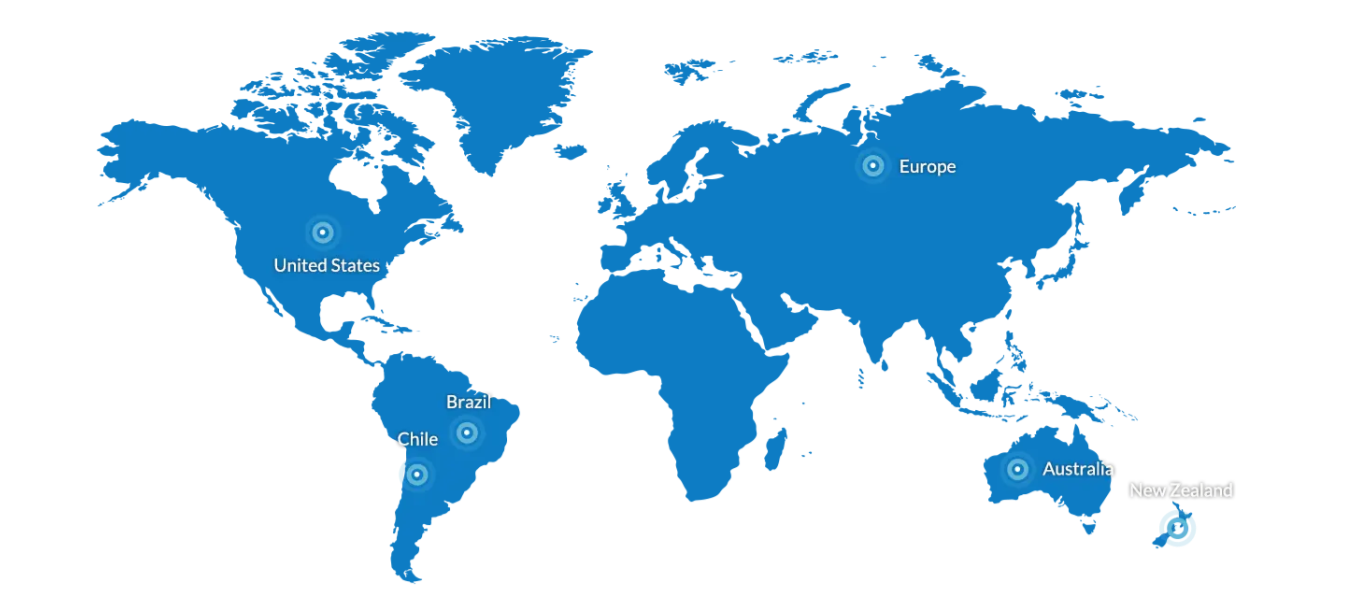
Founded in April 2004 and headquartered in Shenzhen, Shenzhen Huian Bioscitech Co., Ltd. specializes in R&D, production, sales and service of in vitro diagnostic medical devices. As a national high-tech enterprise with complete certifications including NMPA GMP and SGS ISO 13485:2016, it offers the "Q-yianni®" POCT series covering multiple disease diagnostics. Its products are available across 32 Chinese regions and 21 overseas countries/regions, delivering efficient rapid testing solutions globally.

Products
Products

Rapid RSV Ag Test Kit
Professional RSV antigen detection, rapid and accurate diagnosis of respiratory syncytial virus infection

Rapid Malaria P.f./Pan Test
Detects Malaria P.f. and pan-species antigens, used for rapid malaria screening and diagnosis

Rapid Malaria P.f./P.V. Ag
Differentiates Malaria P.f. and P.v. antigens, aids in plasmodium species identification

Rapid Enterovirus Test
Rapid detection of enterovirus, used for diagnosing infections like hand-foot-mouth disease

Rapid H.Pylori Ag Test
Detects Helicobacter pylori antigen, aids in diagnosing H. pylori infection

Rapid H.Pylori Ab Test
Detects Helicobacter pylori antibodies, assesses H. pylori infection status
NUMBER SPEAKS
We always ready for
a challenge.
20000 +
Daily Output
Up to 20,000 units/day (7M+ annually); customized orders may reduce efficiency by 30-35%.
4 +
Core TechsR&D Strength
Leveraging monoclonal antibody & GILFA platform.
48 h
Overseas Response
Remote after-sales support within 48 hours.
1000 +
Industry Recognition
Certified as Shenzhen "Specialized, Refined, Unique & New" SME.
Got an Incredible Project Right Now?
News
Latest news

HUIANBIO Achieves Regulatory Milestone: Helicobacter Pylori Antigen Test Kit (Colloidal Gold) Gains NMPA Approval
September 27, 2025 | Shenzhen, China